Cell Therapy CDMO Services

Offering a Continuum of Cell Therapy CDMO Services
Dedicated Support Every Step of the Way
When you choose OmniaBio as your trusted cell therapy CDMO, you choose a collaborative partnership that prioritizes your project’s uniqueness and success.
Leveraging purpose-built infrastructure, specialized capabilities, and years of experience, we offer tailored support so that you can seamlessly transition your therapeutic program from preclinical to commercial stages.
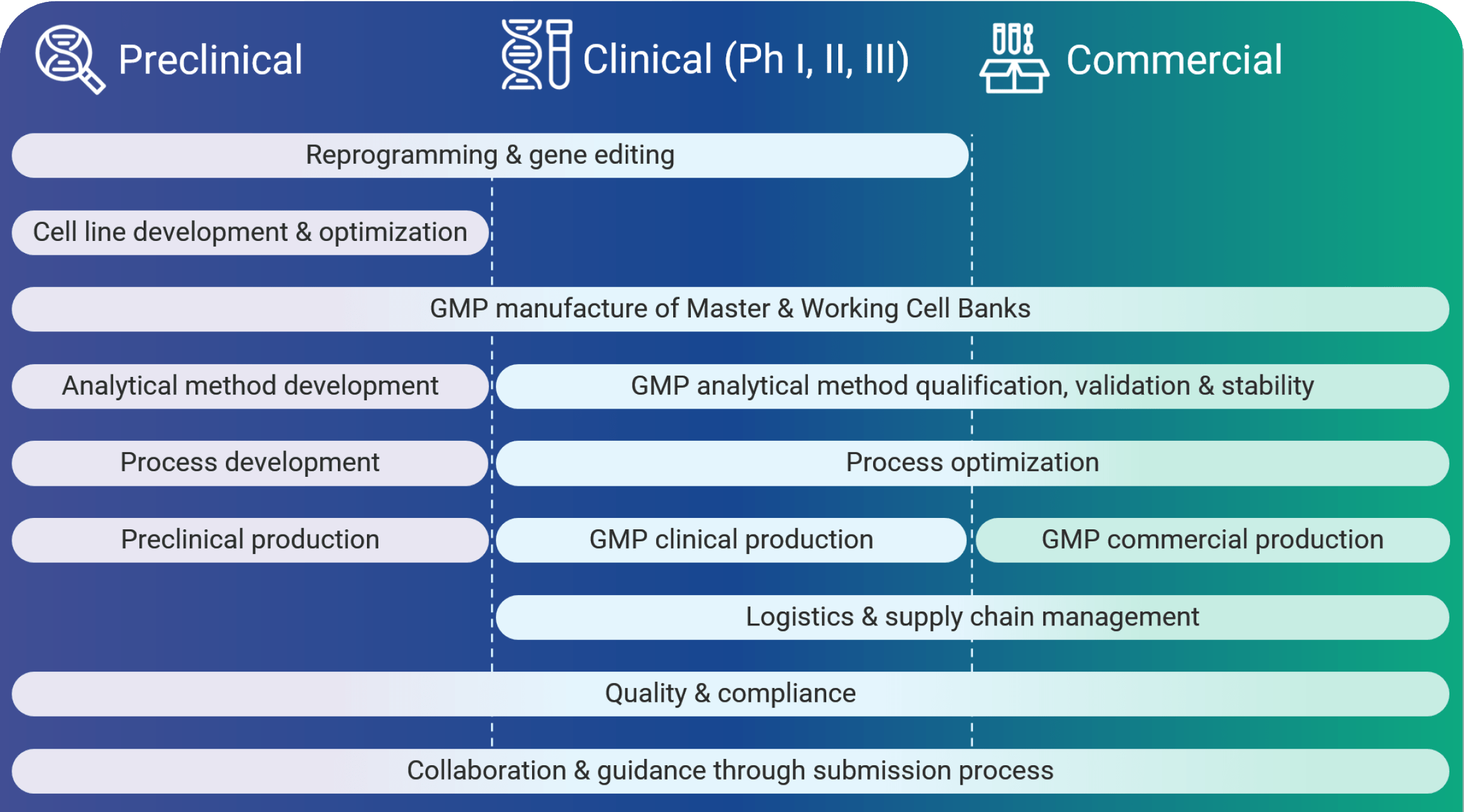
Phase-Appropriate Cell Therapy CDMO Services at Every Stage
From process and analytical development to GMP commercial manufacturing, we support your team at every stage with phase-appropriate services tailored to your therapeutic program needs.
How Can We Help You Meet Your CMC Milestones?
Process Development
Analytical Development
GMP Manufacturing
Platform Expertise

Transforming the Future of Cell Therapy Manufacturing With Purpose-Built Infrastructure
At the forefront of cell and gene therapy manufacturing, our 120,000 ft² Hamilton facility integrates AI models and advanced technologies designed to increase capacity and meet the evolving needs of therapeutic commercialization.
Dedicating 30,000 ft² to GMP manufacturing and operating under stringent quality control, our center of excellence supports OmniaBio’s partners for a smooth transition from early-stage development to commercial stages.
Securing Supply Chain Integrity
Located in proximity to Canada’s largest freight airport and less than 40 miles from the U.S. border, OmniaBio’s infrastructure is strategically positioned to provide flexible logistics solutions for seamless worldwide collection and distribution.
Backed by a 24/7 staffing plan, critical cold chain logistics, and real-time tracking system, we designed our supply chain to meet your manufacturing needs and timelines.

