Secure Your Cell Therapy Supply Chain
Having a robust and efficient cell and gene therapy supply chain for reliably delivering safe and efficacious therapies to patients is paramount. With an experienced supply chain team, strategically located facilities, 24/7 operations, and reputable logistics partners, OmniaBio seamlessly manages all the steps to secure the timely manufacture and delivery of your autologous or allogeneic therapies.

Experienced Supply Chain Leadership
Efficient Vein-to-Vein Logistics

Same day services to anywhere in North America*
Outbound: under 18 hours
Same Day Services to Anywhere in North America*
To DTW
To YYZ
To BUF
To YHM
Outbound: Under 18 hours
Same-Day Service
Flexible Transportation

Temp-Controlled Logistics

Logistics Services Tailored to Meet Your Needs
Compliant Cold Chain Logistics
Specializing in CGT, our reputable partners offer temperature-controlled logistics to ensure the safety and integrity of your cell therapy shipments. Their capabilities include temperature-controlled shippers from ambient to <-150°C / -238°F, temperature monitoring, packaging validation and shipping lane qualification, along with chain of identity and chain of custody management under strict quality control practices.
Expediting Cross-Border Transportation
OmniaBio’s fully trained and dedicated logistics staff manages collection site training, transportation, and administrative paperwork for seamless importing and exporting of your materials and products.
Benefit from expedited border crossings within North America, enabled by our four-step process:
- Preparation: For secure inbound and outbound cross-border transportation, our team conducts pre-shipment preparations, collaborating with North America’s most experienced customs broker to profile your product and materials, ensuring a successful first shipment and setting the standard for subsequent ones.
- Collection Site Training: Before shipment, we train staff at selected collection site(s) on the appropriate method of packaging your material following our SOPs.
- Shipments: We manage your daily logistics in close collaboration with our industry-leading logistics partner to rapidly address any occurrences and prevent impact on your product or timeline.
- Escalation Path: To ensure timely delivery, we have established a robust escalation path involving direct, face-to-face engagement with border agents and officials to resolve logistics issues smoothly.

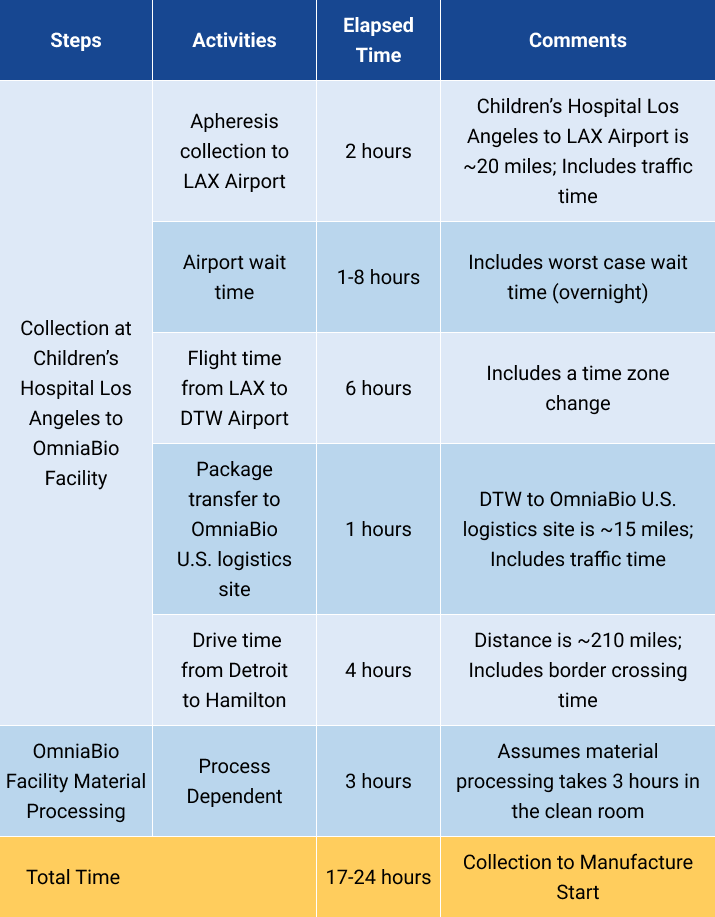
Inbound Case Study
Vein to Manufacture Start: Within 24 hours

| Steps | Activities | Elapsed Time | Comments |
|---|---|---|---|
| Collection at Children's Hospital Los Angeles to OmniaBio Facility | Apheresis collection to LAX Airport | 2 hours | Children's Hospital Los Angeles to LAX Airport is ~20 miles; Includes traffic time |
| Airport wait time | 1-8 hours | Includes worst case wait time (overnight) | |
| Flight time from LAX to DTW Airport | 6 hours | Includes a time zone change | |
| Package transfer to OmniaBio U.S. logistics site | 1 hours | DTW to OmniaBio U.S. logistics site is ~15 miles; Includes traffic time | |
| Drive time from Detroit to Hamilton | 4 hours | Distance is ~210 miles; Includes border crossing time | |
| OmniaBio Facility Material Processing | Process Dependent | 3 hours | Assumes material processing takes 3 hours in the clean room |
| Total Time | 17-24 hours | Collection to Manufacture Start | |
Inbound Case Study
Vein to Manufacture Start: Within 24 hours
Lane Study
| Site | Collection Completed | Destination Airport | Start Time | Packaging | Transit to Airport | Airport Hold | Additional Hold if Required | Total Pre Flight Time | Flight Departure | Flight Arrival | Air Transit Time | Airport Hold | Transit to OmniaBio | Unpack | Total Post Flight Time | Estimated Arrival | Total Transit (Hrs) | Remaining Exploration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LA | PM P/U 4 | Detroit | 4:00 PM | 1 | 1 | 1.5 | 8.5 | 12.00 | 5:30 AM | 1:01 PM | 5 | 1.5 | 4 | 0.5 | 11 | 5:12 PM | 23 | 25.00 |
| PM P/U 4 | Detroit | 4:00 PM | 1 | 1 | 1.5 | 1 | 4.50 | 9:55 PM | 5:22 AM | 5 | 1.5 | 4 | 0.5 | 11 | 10:00 AM | 15.5 | 32.50 | |
| 2:22 PM | 9:49 PM | |||||||||||||||||
| Other Flights | 11:50 AM | 7:14 PM | Other Flights | |||||||||||||||
| 10:19 AM | 5:48 PM | |||||||||||||||||
