Discover Our Technology-Driven Facilities

Same Day Services to Anywhere in North America*
To DTW
To YYZ
To BUF
To YHM
Outbound: Under 18 hours
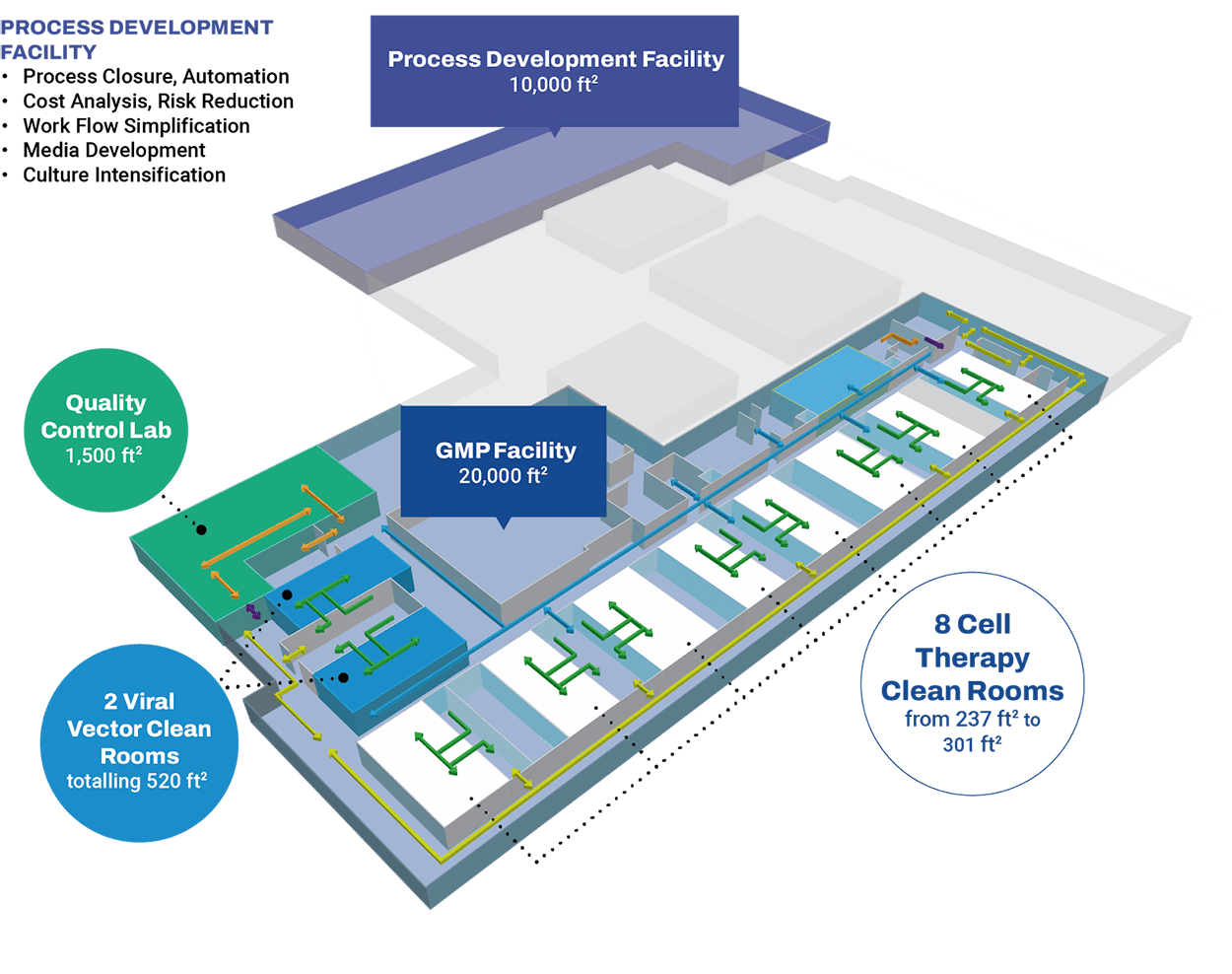
Preclinical & Clinical – Toronto Facility
Our Toronto Partner site, CCRM, is located in the MaRS Discovery District and surrounded by world-renowned clinical and academic facilities. Offering extensive technical expertise and infrastructure, the site efficiently bridges your process and analytical development and GMP manufacturing, de-risking your product’s scaling for faster time to the clinic.
Our Toronto site features:
- Allogeneic and autologous cell therapy manufacturing capabilities
- iPSC, Stem Cell, and LVV manufacturing platforms
- 10 ISO Class 7 Grade B clean rooms
- 10,000 ft2 dedicated to process development
- In-house analytical development and a CL2 quality control lab
- Compliant with US FDA, Health Canada, and European GMP standards
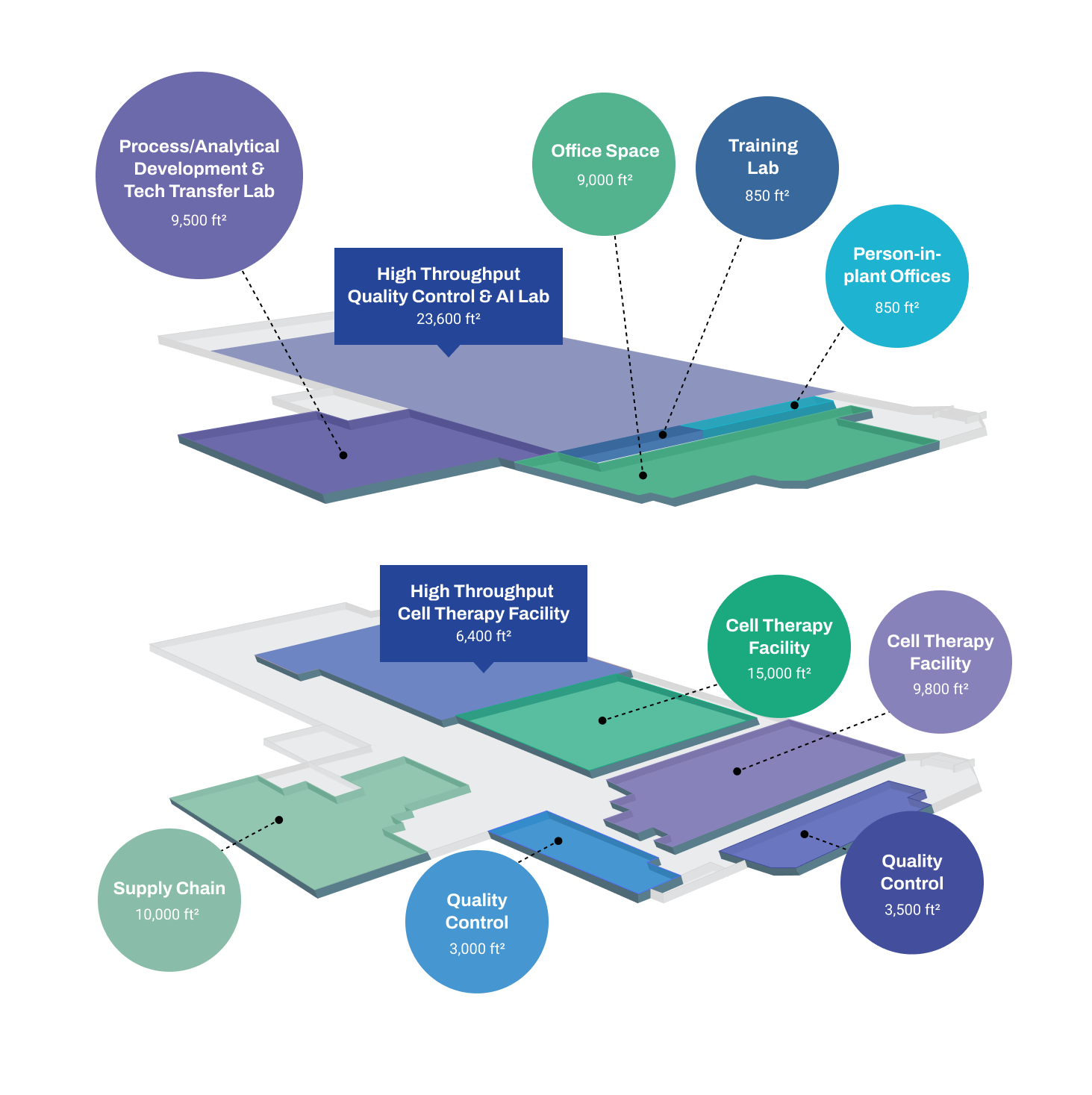
Clinical & Commercial – Hamilton Facility
Built for high throughput, our Intelligent Factory in Hamilton can scale with you from clinical to commercial manufacturing, accelerating time to market. Our Hamilton site is establishing an automation capability that leverages advanced technologies and AI models that help you boost CGT production rates, improve product quality, and lower batch costs.
Our purpose-built site features:
- Allogeneic and autologous cell therapy manufacturing capabilities
- Immunotherapy, iPSC, and Stem Cell manufacturing platforms
- 16 Grade B & C clean rooms for cell processing and F/F
- 9,500 ft2 process/analytical development and technology transfer space
- Two in-house CL2 quality control labs