A Connected Ecosystem from R&D Through Development to GMP Manufacturing
OmniaBio has access to cutting-edge technologies through our parent company CCRM, partnerships with leading equipment manufacturers and supply chain alliances. These relationships ensure we are leading state-of-the-art advancement in iPSC, immune cell-based therapy and lentiviral vector platforms.
OmniaBio has built on the initial seven years of CGT development and manufacturing initiated at our parent company CCRM. Those same experts are now supporting manufacturing at OmniaBio, offering a continuum of cell line development, analytical and process development, GMP manufacture for early Phase I/II, as well as pivotal Phase III and commercial-scale manufacturing.


Compliant Research, Development and Manufacturing Facilities
Building Canada’s Biggest CGT Biomanufacturing Facility

OmniaBio is the anchor tenant in a biomanufacturing centre of excellence at McMaster Innovation Park in Hamilton, Ontario. It will support therapy developers in Canada and internationally by offering both full service and hoteling options, providing access to cutting-edge iPSC, immunotherapy and viral vector manufacturing platforms for cell and gene therapy (CGT).
Industrial-Scale Spaces for Commercial Capacity

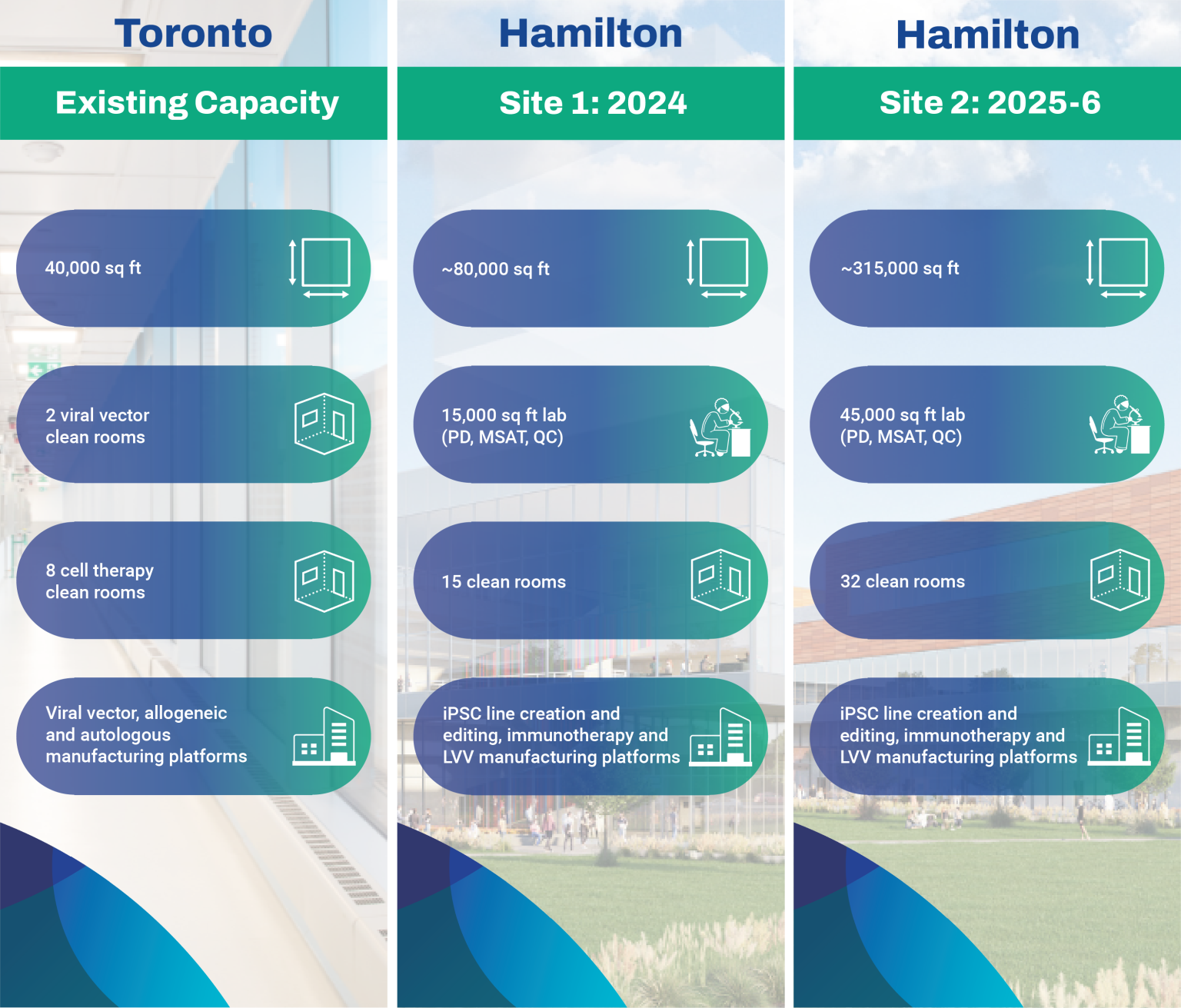
The OmniaBio biomanufacturing sites are opening in a scaled launch between 2024 and 2026, and will total 400,000 sq ft of space adding to the existing 40,000 sq ft of process development and cGMP space at the Toronto site. Boasting multiple viral vector and cell therapy clean rooms for iPSC, LVV and immunotherapy manufacturing platforms, we will be the largest CGT-focused facility in Canada.
Ready for Today, Prepared for Tomorrow
Building for the Future
We’re Growing with the Cell and Gene Therapy Industry
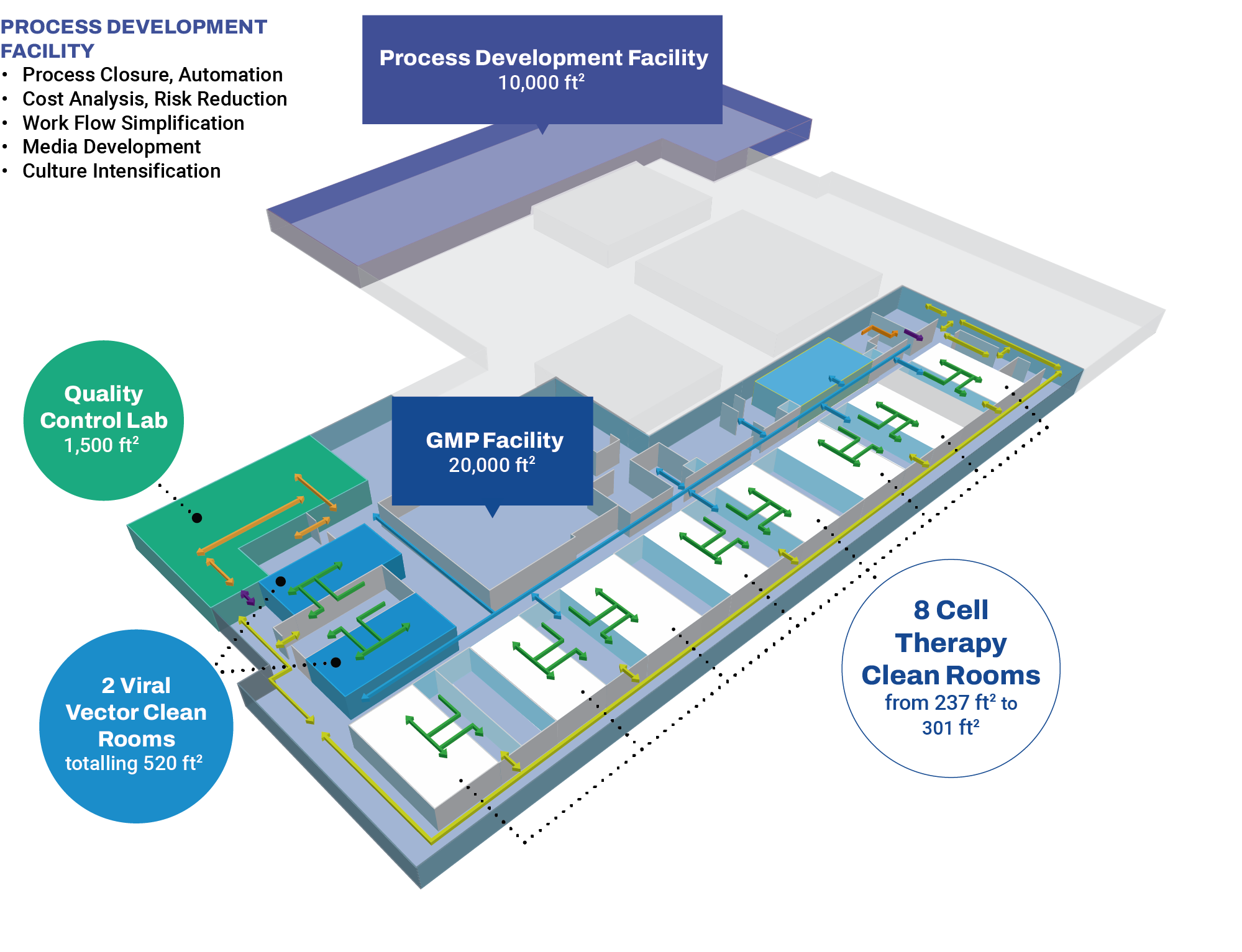
OmniaBio’s early-stage CDMO services occur at the Centre for Cell and Vector Production (CCVP), located within the MaRS Discovery District in Toronto, Ontario. This GMP-compliant facility provides support to therapy developers by manufacturing cells and viral vectors in a 20,000 sq ft laboratory space with 10 clean rooms. To provide seamless continuity for process and analytical development prior to manufacturing, our Centre for Advanced Therapeutic Cell Technologies (CATCT) is co-located within the same facility. OmniaBio will complete CCRM’s continuum of process development and clinical capabilities by expanding the offering to include Phase III and commercial-scale manufacturing in a new biomanufacturing campus located in Hamilton, Ontario.

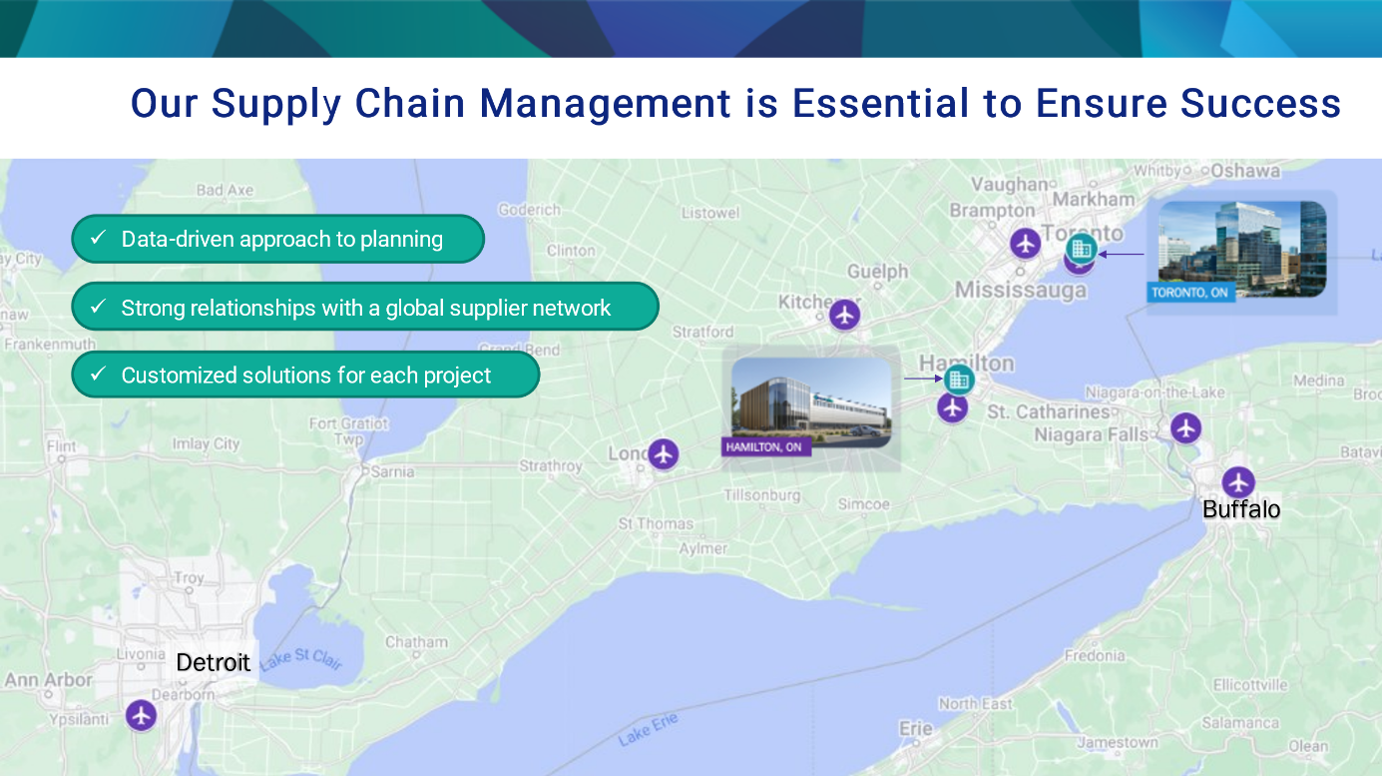
Strategically Located
OmniaBio is located near Toronto and offers smooth and reliable logistics for global shipment, especially to the United States. Our site in Toronto’s Discovery District, near the University of Toronto and more than 30 other institutions, connects us to the third-largest biomedical cluster in North America.